Atomic Number and Mass
Atoms of each element contain a characteristic number of protons and electrons. The number of protons determines an element’s atomic number, which scientists use to distinguish one element from another. The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess. Together, the number of protons and neutrons determine an element’s mass number, as Figure illustrates. Note that we disregard the small contribution of mass from electrons in calculating the mass number. We can use this approximation of mass to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Since an element’s isotopes will have slightly different mass numbers, scientists also determine the atomic mass, which is the calculated mean of the mass number for its naturally occurring isotopes. Often, the resulting number contains a fraction. For example, the atomic mass of chlorine (Cl) is 35.45 because chlorine is composed of several isotopes, some (the majority) with atomic mass 35 (17 protons and 18 neutrons) and some with atomic mass 37 (17 protons and 20 neutrons).
Art Connection
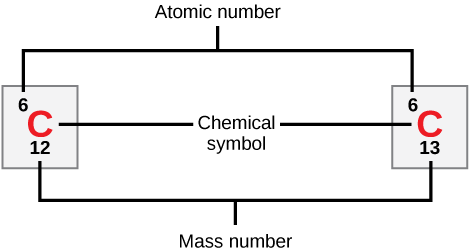
How many neutrons do carbon-12 and carbon-13 have, respectively?