Ions and Ionic Bonds
Some atoms are more stable when they gain or lose an electron (or possibly two) and form ions. This fills their outermost electron shell and makes them energetically more stable. Because the number of electrons does not equal the number of protons, each ion has a net charge. Cations are positive ions that form by losing electrons. Negative ions form by gaining electrons, which we call anions. We designate anions by their elemental name and change the ending to “-ide”, thus the anion of chlorine is chloride, and the anion of sulfur is sulfide.
Scientists refer to this movement of electrons from one element to another as electron transfer. As Figure illustrates, sodium (Na) only has one electron in its outer electron shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. We now refer to it as a sodium ion. Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. We now refer to it as a chloride ion. In this example, sodium will donate its one electron to empty its shell, and chlorine will accept that electron to fill its shell. Both ions now satisfy the octet rule and have complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium cation) or –1 (chloride anion) charge. Note that these transactions can normally only take place simultaneously: in order for a sodium atom to lose an electron, it must be in the presence of a suitable recipient like a chlorine atom.
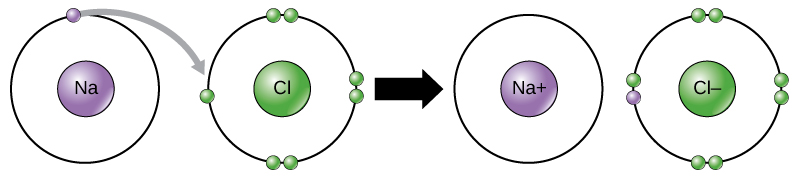
Ionic bonds form between ions with opposite charges. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge.
Physiologists refer to certain salts as electrolytes (including sodium, potassium, and calcium), ions necessary for nerve impulse conduction, muscle contractions, and water balance. Many sports drinks and dietary supplements provide these ions to replace those lost from the body via sweating during exercise.